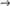medical device replacement parts
Jan . 21, 2025 04:02
Navigating the complex world of medical device replacement parts can be daunting, whether you are a healthcare professional or a procurement specialist. Understanding the intricacies involved ensures operational efficiency and patient safety. The significance of sourcing reliable replacement parts cannot be overstated, as they are crucial for maintaining the functionality of expensive medical equipment such as MRI machines, ventilators, and dialysis units.

Real-world experience shows that timely procurement and installation of high-quality replacement parts directly impact patient outcomes. Facilities that have streamlined their parts replacement process report reduced downtime and enhanced equipment longevity. For instance, a well-maintained CT scanner means fewer disruptions during diagnostic procedures, leading to a more reliable service for both healthcare providers and patients. Those responsible for equipment maintenance often highlight the importance of building relationships with reputable suppliers, ensuring a steady supply of genuine parts and minimizing the risk of counterfeit components.
Expertise in the field is critical when dealing with replacement parts for medical devices. It requires a deep understanding of both the equipment and the components themselves. Technicians must be skilled in installing and calibrating replacement parts to maintain the device's original performance standards. Adequate training and certification programs are essential in cementing this expertise among biomedical engineers and technicians. For example, understanding the exact specifications needed for a replacement part, including material composition and compatibility, ensures the integrity of the device's operation.
medical device replacement parts
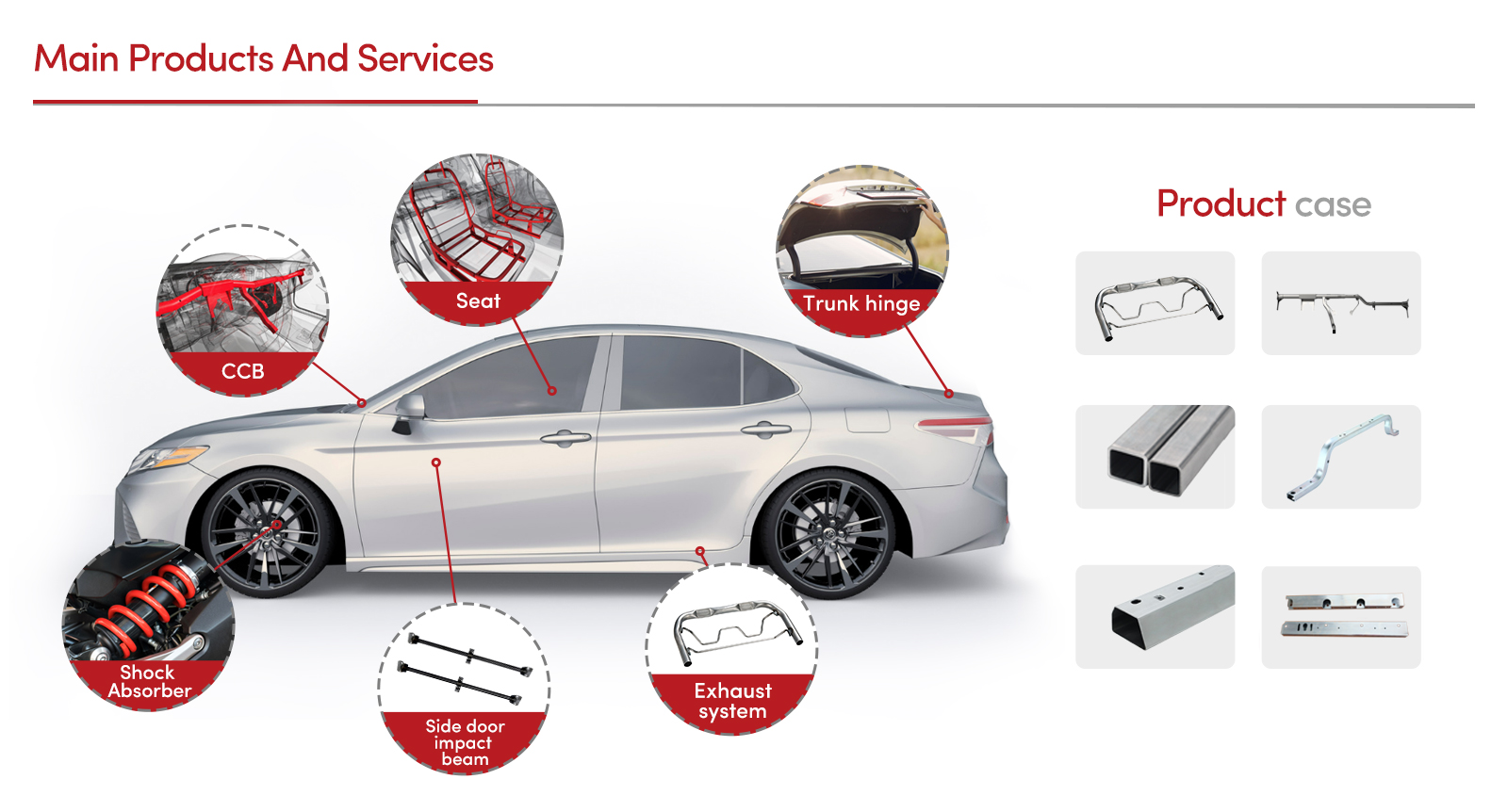
Manufacturers and suppliers need to exhibit authoritativeness by providing comprehensive documentation and support for their replacement parts. This includes offering detailed user manuals, installation guides, and customer support to troubleshoot any issues that might arise. For healthcare facilities, partnering with trusted providers such as those vetted by regulatory bodies like the FDA (Food and Drug Administration) or ISO (International Organization for Standardization) builds a foundation of trust. They not only guarantee that parts meet rigorous safety and performance standards but also offer warranties and after-sales support that enhance reliability.
Trustworthiness is the linchpin in the medical devices industry. Given the stakes involved in patient health, confidence in the source and quality of replacement parts is paramount. Facilities often conduct audits and assessments to ensure suppliers adhere to the highest quality standards. Transparent communication about supply chain practices, sourcing, and sustainability of materials further cements this trust. Furthermore, peer-reviewed studies and endorsements from industry associations can serve as additional validation of a supplier's credibility.
In conclusion, the ecosystem surrounding medical device replacement parts is built on a foundation of experience, expertise, authoritativeness, and trustworthiness. By emphasizing these elements, healthcare providers can ensure that their equipment runs optimally, reducing costs and improving patient care. Meticulous attention to these factors results not only in the smooth operation of critical medical devices but also in robust institutional reputations. Through continuous engagement with trusted suppliers and dedicated training for technical staff, healthcare facilities can navigate the complexities of medical device replacement parts successfully, ultimately contributing to a safer and more efficient healthcare environment.
 Afrikaans
Afrikaans  Albanian
Albanian  Amharic
Amharic  Arabic
Arabic  Armenian
Armenian  Azerbaijani
Azerbaijani  Basque
Basque  Belarusian
Belarusian  Bengali
Bengali  Bosnian
Bosnian  Bulgarian
Bulgarian  Catalan
Catalan  Cebuano
Cebuano  Corsican
Corsican  Croatian
Croatian  Czech
Czech  Danish
Danish  Dutch
Dutch  English
English  Esperanto
Esperanto  Estonian
Estonian  Finnish
Finnish  French
French  Frisian
Frisian  Galician
Galician  Georgian
Georgian  German
German  Greek
Greek  Gujarati
Gujarati  Haitian Creole
Haitian Creole  hausa
hausa  hawaiian
hawaiian  Hebrew
Hebrew  Hindi
Hindi  Miao
Miao  Hungarian
Hungarian  Icelandic
Icelandic  igbo
igbo  Indonesian
Indonesian  irish
irish  Italian
Italian  Japanese
Japanese  Javanese
Javanese  Kannada
Kannada  kazakh
kazakh  Khmer
Khmer  Rwandese
Rwandese  Korean
Korean  Kurdish
Kurdish  Kyrgyz
Kyrgyz  Lao
Lao  Latin
Latin  Latvian
Latvian  Lithuanian
Lithuanian  Luxembourgish
Luxembourgish  Macedonian
Macedonian  Malgashi
Malgashi  Malay
Malay  Malayalam
Malayalam  Maltese
Maltese  Maori
Maori  Marathi
Marathi  Mongolian
Mongolian  Myanmar
Myanmar  Nepali
Nepali  Norwegian
Norwegian  Norwegian
Norwegian  Occitan
Occitan  Pashto
Pashto  Persian
Persian  Polish
Polish  Portuguese
Portuguese  Punjabi
Punjabi  Romanian
Romanian  Samoan
Samoan  Scottish Gaelic
Scottish Gaelic  Serbian
Serbian  Sesotho
Sesotho  Shona
Shona  Sindhi
Sindhi  Sinhala
Sinhala  Slovak
Slovak  Slovenian
Slovenian  Somali
Somali  Spanish
Spanish  Sundanese
Sundanese  Swahili
Swahili  Swedish
Swedish  Tagalog
Tagalog  Tajik
Tajik  Tamil
Tamil  Tatar
Tatar  Telugu
Telugu  Thai
Thai  Turkish
Turkish  Turkmen
Turkmen  Ukrainian
Ukrainian  Urdu
Urdu  Uighur
Uighur  Uzbek
Uzbek  Vietnamese
Vietnamese  Welsh
Welsh  Bantu
Bantu  Yiddish
Yiddish  Yoruba
Yoruba  Zulu
Zulu